HPLC - A method for biochemical research
HPLC stands for High Performance Liquid Chromatography. It
is used for both preparative and analytical material separations. HPLC is
particularly helpful for biochemistry in the preparation and analysis of
substances with high molecular weights such as proteins.
What is liquid chromatography?
Chromatography is a separation process in which a mixture of
substances is separated using two phases, a stationary and a mobile phase. When
talking about liquid chromatography, the stationary phase is a solid and the
mobile phase is a liquid. It is called the eluent.
The mobile phase moves past the stationary phase and the
mixture to be separated is delayed in its passage according to the interactions
between the respective substance and the stationary phase and thus separated.
Various substances can serve as the stationary phase: In
paper chromatography, these are special filter papers; for column and
thin-layer chromatography, silica gel or aluminum oxide are mostly used. The
mobile phases range from non-polar solvents such as hexane to polar solvents
such as water.
How does the HPLC work?
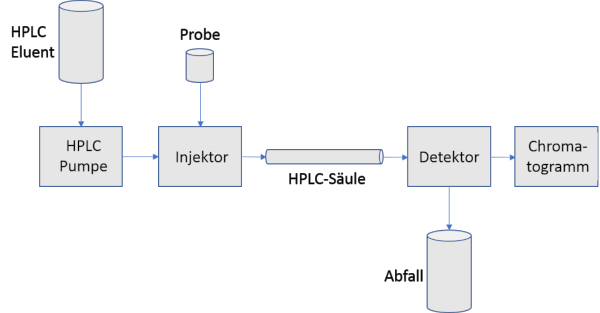
The HPLC is basically a technically optimized column
chromatography. In this separation process, a pump continuously pushes the
eluent, i.e. the mobile phase, through the chromatography column, which is
filled with the solid stationary phase, with as little pulsation as possible at
50 - 400 bar.
If a sample is injected at the beginning of the column with
the aid of an injector, the separation takes place in the column. A downstream
detector measures a signal proportional to the amount of eluted components in
order to then output and qualify this as a graphic, the so-called chromatogram.
In the ideal case, each peak of a chromatogram represents a component of the
mixture of substances
Common detectors are, for example, UV absorption, refractive
index, or fluorescence detectors. By returning pure eluents that are not
contaminated with the sample, the solvent consumption of such a system can be
reduced significantly.
In contrast to simple column chromatography, which works
without pressure, with high-performance liquid chromatography the sample
quantities are significantly smaller, the elution speed is significantly higher
and the separation efficiency is much better and stationary phase and thus
affect the separation efficiency of the column
In devices for ultra-high-performance liquid chromatography
(UHPLC), even smaller particles and, at the same time, even shorter and thinner
columns are used, thus further optimizing the separation performance.
In the following, we briefly discuss the usual components of
an HPLC
Sample injection
The dissolved sample is usually injected with the aid of
sample loops with a defined volume, into which the sample is first introduced.
After switching, a six-way valve enables the eluent to flow through the loop
loaded with the sample, and thereby the sample is applied to the column.
In the low and medium pressure range, sample injection using
a dosing syringe is also possible. In routine operation, an autosampler reduces
the manual workload. The injection volumes depend on the column diameter and
are usually only 1 µl to 2 ml.
Since high pressures of up to 400 bar are used, columns made
of thick-walled glass are used, which for safety reasons are usually encased in
a pressure-resistant stainless steel tube. High pressure-resistant columns made
of stainless steel, however, can hardly be used for biochemical work because of
the sensitivity of many bioactive substances to heavy metals. Occasionally,
however, pillars made of bio-inert titanium are also used. The effective inside
diameter of HPLC columns is usually only a few millimeters.
Stationary phase
HPLC columns are tightly packed with a solid but porous and
pressure-resistant material, usually a functionalized silica gel. Compared to
normal liquid chromatography in the low-pressure range, the particle size of
the stationary phase in HPLC is considerably smaller at <10 μm. The smaller
the particles, the higher the separation efficiency.
The sample can be separated according to various principles
such as adsorption, ion exchange, and size exclusion chromatography, thus
opening up a wide range of possible applications.
Usually, the separation takes place due to the polarity
differences between the mobile and stationary phases. A distinction is made
between normal-phase HPLC with a polar stationery and a non-polar mobile phase
(NP) and reversed-phase HPLC (RP) with correspondingly reversed polarities.
The polarities of the stationary and mobile phases have a
considerable influence on the retention time, the transit time of a substance
through the column. A substance has no or only very weak interactions with a
non-polar stationary phase and therefore has a short retention time.
However, since normal phase HPLC is more difficult to use,
e.g. Due to problems caused by water in the solvent, for example, reverse phase
HPLC is the method that is used much more frequently today.
Mobile phase
When selecting the eluent, polarity overviews or elutropic
series can help. For a large number of mixtures, solvent mixtures are required
with which the polarity and thus also the elution power can be set in a
targeted manner. Electronically controlled mixers for the solvents can be used
to set the polarity gradients of the eluent.
Range of application of high performance liquid
chromatography
Analytically
A substance is usually identified analytically by comparing
the retention times of the sample with that of a reference substance. For
example, you can mix the sample and comparison and inject them together; an
enlarged peak should then result. However, if a second peak is eluted, the
sample and reference substance are not identical. By repeating the analysis
after changing the mobile phase, it can be ensured as far as possible that the
peaks are not just randomly superimposed.
Changing the stationary phase (e.g. reversed phase instead
of normal phase HPLC) brings even greater security. One hundred percent
certainty, however, can only be obtained by qualitative analysis of the
substance for the respective signal. This can also be achieved with specific
downstream detectors, such as mass spectroscopy or diode array, with which the
entire UV / Vis spectrum of a signal is recorded.
Preparative
Just like normal column chromatography, HPLC can also be
used to purify substances such as pharmaceutical products or biological
samples. As a rule, columns with larger internal diameters are required for
this, because the quantities of samples to be purified are usually many times
greater than in the case of analytical separations.
Importance in biochemistry
Due to a large number of different separation principles and
a wide variety of detectors, high-performance liquid chromatography is a very
versatile and powerful analysis tool, especially in biochemistry, where
mixtures of biopolymers such as nucleic acids, amino acids, peptides, or
enzymes have to be identified. If the columns are dimensioned accordingly, the
HPLC is also suitable for preparative separations for the purification of substances
from mixtures. It has thus become indispensable in the biochemical laboratory
in particular.
High-performance liquid chromatography is also used today
for medical purposes, such as determining the vitamin D content in the blood.
Other typical applications include the analysis of polymers,
active ingredients, and pollutants, purity controls or quantity determinations,
e.g. B. of active ingredients in biological samples. HPLC analysis is also used
as standard today in drinking water control.
Comments
Post a Comment